Serving Southern California and Northern California
Southern California
Northern California
Serving Both Southern and Northern California
Heart Treatments We Offer at Our San Diego and Redding Locations
Ultra-Sensitive CRP
Clinical cardiologists well know the fact that up to 40% of patients with documented coronary artery disease (CAD) have normal cholesterol values. The cholesterol and lipid assay, though helpful, is not sensitive enough to detect abnormalities in lipid metabolism at the level of the coronary artery. Since 1995, studies have pointed to an inflammatory component as a cause of CAD. Some agent causes the cells that make up the cell wall of the coronary artery to become inflamed or irritated. Some think bacteria or viruses incite this inflammatory process. Whatever the cause, the body notices the damage and sends specialized blood cells to destroy the foreign substance and then repair the damage. However, this process itself causes further compromise of the coronary artery lumen. The damage culminates in a tear or dissection of the coronary artery wall. This releases the lipids within the wall. The blood cells, when it detects the lipids released into the blood, respond by clotting. This culminates in blockage of the entire lumen resulting in a heart attack.
Yet in 40% of people with this process going on, have “normal” cholesterol values. Dr. Richter in 1997 published in the New England Journal of Medicine a common assay used to measure inflammation—the CRP (C-Reactive Protein)—if measured in tiny increments can detect ongoing coronary artery inflammation even when cholesterol values are normal. CRP in medical terms is called an acute phase reactant: a protein in blood that rises when inflammation or infection is actively going on. CRP has been used by doctors since 1950s to measure progress of antibiotic treatment on an infection. If the CRP is going down, then the antibiotic regimen is working. If the CRP continues to rise, the antibiotic regimen chosen is not working and a change is warranted. CRP is usually measured 1-500 pg/ml.
Dr. Richter used CRP not in the 100s but only in the range of 0-5 pg/ml. This Ultrasensitive CRP was measured in patients with already documented CAD. He noticed that people who have documented CAD and have normal cholesterol values who also have elevated values of Ultrasensitive CRP went on to have future coronary events. Those patients with CAD with normal cholesterol values who have low Ultrasensitive CRP tended not to end up having as many coronary events. He, therefore, recommended patients with documented CAD to be followed with Ultrasensitive CRP especially when they have normal cholesterols. What is not known is if the Ultasensitive CRP is brought down, does this then reduce the chances of the coronary event occurring in the future? This will need to be proven with clinical research.
Dr. Nanavati has been using Ultrasensitive CRP assay on all his patients with CAD since 1999.
Coronary Stenting
Stents are metallic hollow devices that serve to scaffold an area of obstruction within the coronary artery. The stents made today are 2.0 to 4.5 mm in diameter and vary in length from 5 mm to 40 mm. Most stents are made of stainless steel.
The first stent was successfully implanted in a human in 1993 in Europe. Shortly thereafter in 1994, the first stent was successfully implanted in the U.S. Then followed two large, randomized trials: STRESS and BENESTENT. These two trials the former in the US, the latter in Europe, showed that stents placed in coronary arteries were better than conventional balloon angioplasty (PTCA) in maintaining vessel patency. The restenosis rates were 17-25% for stents while PTCA restenosis rates were 35%.
In 1995, when stents were first available for clinical use, stent implantation was fraught with two major problems:
1. Large doses of blood thinners were required leading to unacceptable high rates of bleeding. If the blood thinner was not used, stents quickly clot off resulting in large disastrous heart attacks!
2. Long hospitalization rates two to three times that of conventional PTCA.
Both these limitations were overcome when it was found that Ticlid was far superior that Coumadin in preventing acute stent occlusion from clot. Ticlid also had much lower rates of bleeding compared to Coumadin. Clopidogrel, a drug similar in molecular structure to Ticlid but with fewer side effects, is used instead of Ticlid. With the replacement of Ticlid or Clopidogrel instead of Coumadin, patients’ rates of excessive bleeding fell dramatically. As a direct consequence, the duration of hospital stays also fell dramatically. Now days, patients usual stay in the hospital for only one day after an uncomplicated stent deployment. With the use of Ticlid or Clopidogrel, acute stent occlusion is a now a rare phenomenon.
Narrative Of Stent Procedure
What about restenosis? Restenosis is the gradual process by which the stented or ballooned area gets plugged up by tissue ingrowth and plaque. The rates of restenosis for conventional angioplasty are 40% in four to six months after the PTCA. In BENESTENT and STRESS trials, the restenosis rates were 17 and 25% respectively. With the newer generation stents, restenosis rates have fallen to 10 to 15% in national registries. In order to get around this challenge of stent restenosis, newer Drug eluting stents were designed. In 2003, two drug eluting stents were introduced: Cypher which elutes the drug Sirulimus and Taxus which elutes the drug Paclitaxel. After placing these types of drug eluting stents (DES) the stent restenosis rates fell dramatically down to single figures. As of this update, in 2012, there are now three new drug eluting stents available eluting two different drugs: zotarulimus, and Evarilimus. Both these drugs are variants of the Sirulimus drug from the initial Cypher stent. Stent restenosis is reduced but not totally resolved by implantation of Drug Eluting Stents. There is also procedural differences in how the stents are implanted by the Cardiologist. Before getting your stent procedure, you should ask your cardiologist for his/her specific restenosis rates.
Stent deployment in a coronary artery has made it possible for most patients to avoid a disastrous heart attack. However, neither stent deployment nor any other intervention can reverse the atherosclerosis process. Atherosclerosis is the abnormal accumulation of cholesterol and lipids in the walls of blood vessels. Atherosclerosis, if left untreated, can culminate in a heart attack.
Reversing the process requires changing your lifestyle: increasing activity, lowering the fat intake in your diet and reducing your blood cholesterol. Read more about how you can prevent a heart attack here.
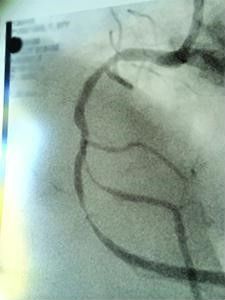
BEFORE
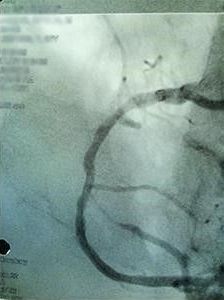
AFTER STENT
"To view more results of Dr. Nanavati's work, please go to "Gallery" page on this website or visit IG @heartdoc530
Precautions After Intracoronary Stent Implantation
If you have recently received or are about to receive a stent implanted in your coronary artery, please read and follow the following precautions.
1. Do not stop Plavix (Clopidogrel) or Ticlid (Ticlopidine) for any reason. If you received a Bare metal stent, you must continue one of these two important anti-platelet medications for at least 4 weeks after stent implantation. You cannot receive any elective surgery for at least 6 weeks of continuous daily Plavix or Ticlid.
2. If you received a Drug Eluting stent, no elective surgery can be recommended for at least one year. In fact, Plavix or Ticlopidine cannot be stopped even for one day and must be continued for at least one year.
3. Use Antibiotics prior to dental or any endoscopic procedures for the first 3 months after stent implantation.
4. Avoid MRI for 8 weeks after stent implantation.
5. If you have any chest discomfort that does not resolve after sublingual Nitroglycerin used three times in a row, call 911 and have the Emergency Room MD contact your Cardiologist. When you take the Nitroglycerin, make sure you wait at least five minutes between doses. Taking too much Nitroglycerin can cause your blood pressure to drop and then you could pass out.
6. Keep a wallet-sized card which contains all the necessary information regarding your stent:
– Name and size of stent.
– Type of stent (Bare metal or Drug eluting)
– Location of stent(s) in the coronary vessel.
– Name of implanting Cardiologist.
– Phone number of implanting Cardiologist.
– Date of stent implantation.
Typically, the stent manufacturer provides such cards for your use after the stent is implanted. If you did not receive this card at the time of implantation, call your Cardiologist: he or his staff will make sure you receive this valuable information. Make sure you keep this card in your wallet or purse at all times.
Radial Artery Catheterization
Watch Dr. Vimal Nanavati discuss Radial Artery Catherization below.
How Are Coronary Stents Deployed
Stents are metallic slotted tubes that are placed at the site of a coronary blockage to open up the inside of the artery to allow for increased blood flow through the artery. These metal tubes are crimped onto a balloon. The balloon with the crimped stent on it are then advanced over a wire (like a train on tracks). Once the stent reaches the area of blockage (or stenosis), the balloon is expanded so the stent gets deployed in the artery. In order to be effective, the stent must be fully touching the internal artery wall on all sides. The stent is permanent. It never comes out. Although its metallic, stents don’t set off metal detectors at security screening stations and are safe from MRI after 6-8 weeks of initial implantation.
Background of Coronary Stenting
Stents were designed because balloon angioplasty (PTCA) was fraught with two major problems:
1. The area that was opened by balloon angioplasty abruptly closes up.
2. The ballooned area is incomplete opening and if the balloon is expanded too vigorously, the arterial wall is torn causing a dissection.
In the early 90s, stents were introduced. Initial deployment of stents were plaque by excessive clotting and bleeding. The agent initially used to keep stents open was Coumadin and Aspirin. This turned out to cause excessive bleeding at puncture sites but the combination didn’t prevent the stents from clotting off. Finally around 1995, Ticlopidine, an new antiplatelet drug, was introduced. Ticlopidine and Aspirin turned out to be the correct combination that didn’t lead to excess bleeding and allowed the stent to not clot off.
Around the same time, Dr. Antonio Colombo made a very interesting observation that would change the way we implant stents forever leading to durable excellent stent results. Using intracoronary unltrasound or IVUS (a catheter that can be advanced within a coronary artery that has an ultrasound probe at the tip allowing for ultrasound pictures of the internal part of the coronary artery), he noticed that most of the stents he implanted were incompletely expanded and failed to touch the internal wall of the coronary artery. He then went back and reintroduced balloons at high-pressures to achieve proper expansion of the recently implanted stent; this lead to little to no stent blockage or clotting off of the stents.
It is these two developments that made stent implantation a practical solution to abrupt closure issue of the PTCA as well as sealing any dissection or tear of the artery. Stents implantation then took off and quickly supplanted PTCA as the primary method of opening blocked coronary arteries.
Ticlopidine was replaced in 1998 by Clopidogrel, an agent that did not have the side effects of Ticlopidine. Clopidogrel was much better tolerated and also essentially supplanted Ticlopidine as the adjuvant antiplatelet drug necessary after stents were implanted. There are newer drugs now on the market that can be used in replacement of Clopidogrel: Ticagrelor and Prasiguel. Both these drugs are more potent than Clopidogrel. These antiplatelet drugs have come to be known as P2Y12 inhibitors. These P2Y12 inhibitors must be taken daily as prescribed or else the patient has a risk of the very disastrous complication of sudden and complete closure of the stent with clot.
As stent implantation became commonplace, problems with stent implantation was noticed. Principally, metal stents tended to cause an over-zealous response of surrounding smooth muscle cells which if continuing to multiple, would in itself block the flow. Ideally, a single layer of cells were supposed to cover (endothelialize) the metallic surface of the stent so as to allow for stopping the antiplatelet drugs. It turns out that the process of endothelialization is complete after 4 weeks. However, in many patients, smooth muscle cell proliferation was so vigorous that the cells themselves created a blockage where the stent had been placed. There was no good solution to this process of excessive smooth cell proliferation, until new stents were developed. These new Drug Eluting Stents(DES) had drugs which combatted the smooth muscle cell growth impregnated within a collagen scaffold that surrounded the metal of the stent struts. The scaffold allowed the drug to slowly diffuse into the surrounding smooth muscle in contact with the stent so that the smooth muscles wouldn’t excessively react to the stent scaffold. While this did solve the problem of over vigorous cell growth and subsequent blockage, it also caused incomplete coverage of the metal in the long term (beyond one year). This lead to the metal being exposed to blood thus resulting in blood clots (the same problem that plagued the early stent implanters). Blood when it comes into contact with metal or any foreign object will clot.
This lead to the development of two new stent designs:
1. Bio-absorbable stent platforms that are not made of metal but instead are made of a different substance that dissolves over time. So the stent struts actually dissolve and disappear.
2. Metal stents impregnated in drug that prevent smooth muscle cells from proliferating.
Both stent designs are currently under research evaluation. While stent designs are constantly evolving, the actual procedure has remained relatively the same. (see How Stents are deployed). It turns out that devices used to image the inside of the artery (IVUS and OCT) have found the newly developed implanted stents were still underexpanded (sometimes even floating in the coronary lumen). Until the we continue to refine the technique of stent implantation, no matter what the design advancement, we may still have problems with stents coming back blocked or clotted off.
Despite these continuing issues, stent implantation has become the standard way Cardiologists deal with coronary artery blockages in one or two vessels. They have become a life-saving procedure in people having an acute heart attack.
Daily Aspirin: Abandon its Recomendation?
Almost 30 million Americans take aspirin for primary or secondary prevention of cardiovascular disease (CVD) or colorectal cancer (CRC).1 For decades, aspirin has been recommended as primary prevention for patients at risk for CVD, despite the attendant increased risk of major bleeding.2 However, the results of recent clinical trials have challenged the clinical risk-to-benefit trade-off of daily aspirin use.
The most recent recommendations from the US Preventive Services Task Force (USPSTF) have abandoned the routine recommendation for daily aspirin use for primary prevention of CVD.3 Continue reading to learn more about the potential benefits and harms of daily aspirin use.

What Are the Potential Benefits of Daily Aspirin?
Aspirin has been used for its antipyretic and analgesic properties long before it came into use to prevent or treat CVD. After the discovery of aspirin’s antiplatelet and antithrombotic effects, researchers began to explore the possibility of using it to prevent and treat acute coronary syndrome and stroke.
At low doses, aspirin is an irreversible inhibitor of cyclo-oxygenase-1 (COX-1), which inhibits platelet function.3 In patients with pre-existing CVD, aspirin can reduce the risk of experiencing a CVD event by 21% and all-cause mortality by 13%. However, the benefit of daily low-dose aspirin use in patients without pre-existing CVD (primary prevention) is less clear.
Although the systematic review commissioned by the USPSTF found that daily low-dose aspirin is associated with a clinically significant reduction in absolute risk of CVD events, it is a modest reduction, with an odds ratio (OR) of 0.9.
Daily Aspirin Use: Current USPSTF Recommendations to Prevent Cardiovascular Disease
New studies have highlighted the potential for daily aspirin to cause harm. In 2022, the USPSTF released new recommendations on the use of aspirin for the primary prevention of CVD. The new recommendations are as follows:
- For adults between 40 and 59 years of age with a 10% or greater 10-year CVD risk, the Task Force recommends that the decision to start aspirin therapy be an individual one. This recommendation is offered as a grade C recommendation, with moderate certainty of a small benefit.
- For adults older than 60 years, the Task Force recommends against starting low-dose aspirin therapy. This recommendation is offered as a grade D recommendation, which recommends against using aspirin for this population.
These recommendations apply to adults older than 40 years of age without signs or symptoms of CVD and not at increased risk for bleeding.
What Changed From the Previous USPSTF Recommendations?
The 2016 USPSTF recommendations had stronger recommendations for the use of aspirin to prevent CVD and CRC. The new recommendations concluded that there was insufficient evidence that low-dose daily aspirin reduces the incidence of or mortality from CRC. Therefore, the new recommendations only address daily aspirin use for the primary prevention of CVD.
The previous recommendations advised initiating daily aspirin use for adults aged 50 to 59 years with a 10% or greater 10-year CVD risk without increased risk for bleeding, who have a life expectancy of 10 years or greater, and who are willing to take daily low-dose aspirin for at least 10 years. This recommendation was also a higher grade (Grade B — high certainty of a moderate benefit) compared with the updated recommendations.
In the 2016 USPSTF recommendations, the Task Force recommended an individual decision for initiating aspirin use for adults aged 60 to 69 years with a 10% or greater 10-year CVD risk. This recommendation was offered as a grade C recommendation. The previous recommendations cited insufficient evidence for daily aspirin use in adults younger than 50 years and older than 70 years. The updated recommendations have addressed patients as young as 40 years.
Why Did the USPSTF Recommendations Change?
New data on the potential risk of aspirin have prompted the change in the USPSTF recommendations on daily aspirin use for primary prevention of CVD. The primary risk of daily aspirin use is an increased risk of major bleeding such as gastrointestinal bleeding, intracranial bleeding, and hemorrhagic stroke.
The factor contributing to the increased risk of bleeding is aspirin’s inhibition of platelet activity. Additionally, by inhibiting COX-1, aspirin may promote gastrointestinal bleeding by inhibiting the production of several prostaglandins that protect the gastrointestinal mucosa.
Since the 2016 USPSTF recommendations, 3 trials have been published with a focus on special populations of patients, including those with older age, diabetes, and additional CVD risk factors.
Aspirin Use in Older Adults
A randomized, placebo-controlled clinical trial (ASPREE; ClinicalTrials.gov Identifier: 01038583) of more than 19,000 adults older than 70 years in Australia and the United States found that, compared with placebo, enteric-coated aspirin 100 mg resulted in a significantly higher risk of major bleeding. Additionally, aspirin 100 mg/d was not associated with a significantly lower risk of CVD compared with placebo.
Aspirin Use in Adults With Diabetes
A randomized, placebo-controlled trial (ASCEND; ClinicalTrials.gov Identifier: NCT00135226) of more than 15,000 adults with diabetes but without CVD found that, compared with placebo, aspirin 100 mg/d significantly reduced the risk of CV events (8.5% in the aspirin group vs 9.6% in the placebo group; P=.01). However, the authors concluded that these benefits were counterbalanced by the increased risk of bleeding (4.1% in the aspirin group vs 3.2% in the placebo group; P=.003).
Aspirin Use in Adults at Moderate Risk for CVD
The Aspirin to Reduce Risk of Initial Vascular Events (ARRIVE; ClinicalTrials.gov Identifier: NCT00501059) study was a randomized, placebo-controlled trial of more than 12,000 adults at moderate risk for CVD (defined as a 10% to 20% 10-year risk). Due to a lower-than-expected event rate, the authors were unable to address the role of aspirin in the primary prevention of CVD in a moderate-risk population. However, the results were consistent with the results from other trials with a low-risk population.
Updated Evidence Report and Systematic Review for the USPSTF
The systematic review commissioned by the USPSTF included 11 randomized controlled trials (including the studies discussed above) and 1 pilot trial with more than 134,000 patients. The authors of this systematic review found that although low-dose aspirin was associated with a small reduction in CV events, it was also associated with small increases in major bleeding events.4
The results for aspirin use for the prevention of CRC were not as robust as those for primary CVD prevention and were highly variable.
Authors of another systematic review and meta-analysis from 2019 on the association of aspirin use with CV events and bleeding events found that the absolute risk reduction of CV events (0.41%) did not outweigh the increased risk of major bleeding (0.47%).
Implementing USPSTF Daily Aspirin Recommendations Into Practice
The 2022 USPSTF recommendations align with the position of other professional organizations and guidelines, including11-13:
- 2019 American College of Cardiology/American Heart Association (ACC/AHA) Guideline on the Primary Prevention of Cardiovascular Disease — available here11; and
- 2021 European Society of Cardiology (ESC) Guidelines on Cardiovascular Disease Prevention in Clinical Practice — available here.
The American Academy of Family Physicians (AAFP) has also released a statement supporting the USPSTF recommendations on aspirin use for the primary prevention of CVD.
Shared Decision-Making
Aspirin use is no longer routinely recommended for any patient. Initiating daily aspirin use should be based on shared decision-making between patients and their health care providers. It is important to determine what factors are most important for patients when making this treatment decision.
Patients who choose to initiate aspirin may place a higher value on aspirin’s benefit of decreasing the risk of CV events and stroke than the increased risk of bleeding. For these patients, clinicians can recommend low-dose aspirin (< 100 mg/d). The most common aspirin dosage for this purpose is 81 mg/d.
Patients who choose not to initiate aspirin may place a higher value on the increased risk of bleeding and the increased pill burden of daily aspirin.
Clinicians should educate patients about the increased risk of bleeding, including signs and symptoms to watch for, such as3,14,15:
- Severe headache;
- Dizziness;
- Nausea and vomiting;
- Seizures;
- Weakness on 1 or both sides;
- Fatigue;
- Aphasia;
- Black, tarry stool;
- Coffee ground vomit;
- Abdominal cramping; and
- Pallor.
Re-evaluating Patients Taking Daily Aspirin
Patients currently taking aspirin daily should be regularly evaluated to ensure the benefit of treatment still outweighs the risk. The risk of serious bleeding increases with age. Modeling data from the USPSTF shows that it may be reasonable to stop daily aspirin use at the age of 75 years.
Alternative Approaches to Primary CVD Prevention
Other approaches to the primary prevention of CVD should also be considered. Other therapies with a more favorable safety profile than aspirin include antihypertensive agents and statin therapy.
In the future, it may be possible to identify distinct populations of patients that may benefit from aspirin therapy, such as those with hyperactive platelets. However, more research is needed to determine the potential benefit to these patients.
This article originally appeared on The Cardiology Advisor.
References
- Cofer LB, Barrett TJ, Berger JS. Aspirin for the primary prevention of cardiovascular disease: Time for a platelet-guided approach. Arterioscler Thromb Vasc Biol. 2022;42(10):1207-1216. doi:10.1161/ATVBAHA.122.318020
- Fuster V, Sweeny JM. Aspirin: a historical and contemporary therapeutic overview. Circulation. 2011;123(7):768-778. doi:10.1161/CIRCULATIONAHA.110.963843
- US Preventive Services Task Force. Aspirin use to prevent cardiovascular disease: US Preventive Services Task Force recommendation statement. JAMA. 2022;327(16):1577-1584. doi:10.1001/jama.2022.4983
- Guirguis-Blake JM, Evans CV, Perdue LA, Bean SI, Senger CA. Aspirin use to prevent cardiovascular disease and colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2022;327(16):1585-1597. doi:10.1001/jama.2022.3337
- Aspirin use to prevent cardiovascular disease: preventive medication. US Preventive Services Task Force. Published April 26, 2022. Accessed August 17, 2023.
- Aspirin use to prevent cardiovascular disease and colorectal cancer: preventive medication. US Preventive Services Task Force. Published April 11, 2016. Accessed August 17, 2023.
- McNeil JJ, Wolfe R, Woods RL, et al; ASPREE Investigator Group. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509-1518. doi:10.1056/NEJMoa1805819
- ASCEND Study Collaborative Group; Bowman L, Mafham M, Wallendszus K, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379(16):1529-1539. doi:10.1056/NEJMoa1804988
- Gaziano JM, Brotons C, Coppolecchia R, et al; ARRIVE Executive Committee. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036-1046. doi:10.1016/S0140-6736(18)31924-X
- Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta-analysis. JAMA. 2019;321(3):277-287. doi:10.1001/jama.2018.20578
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646.
- Visseren FLJ, Mach F, Smulders YM, et al; ESC Scientific Document Group. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227-3337. doi:10.1093/eurheartj/ehab484
- Aspirin use to prevent CVD and colorectal cancer – clinical preventive service recommendation. American Academy of Family Physicians. Accessed August 18, 2023.
- Hemorrhagic stroke. Cleveland Clinic. Updated August 7, 2022. Accessed August 18, 2023.
- Gastrointestinal (GI) bleeding. Cleveland Clinic. Updated June 27, 2022. Accessed August 18, 2023.
Discuss With Us the Heart Treatment that is Right For You. Call For an Appointment at our Redding Office Today at 530-433-5427 and our San Diego Office at (619) 585-0476.
SITE LINKS
CONTACT INFORMATION
Southern California
Northern California